Aptima® Vaginal Health
Aptima Vaginitis Panel
The Aptima vaginitis panel from Hologic consists of molecular nucleic acid amplification tests (NAAT) to aid in the detection of vaginosis and vaginitis. Our assays detect the three most common causes of infectious vaginitis: bacterial vaginosis, candida vaginitis and trichomoniasis.
Vaginitis is a common problem that affects millions of women.
Ten million women each year visit their healthcare providers seeking a cure for vaginitis and a third of all women will have symptoms of vaginitis at some point during their lives, most commonly during their reproductive years. Some types of vaginitis are transferred through sexual activity, while others arise because of an imbalance in the bacterial or fungal makeup of the vaginal microbiome.3 Combined, bacterial vaginosis (BV), candida vaginitis (commonly known as a yeast infection, CV), and trichomoniasis (Trichomonas vaginalis, TV) make up 90% of vaginitis cases.
Hologic is an innovative medical technology company focused on improving women’s health, and features two assays that aid in diagnosing BV, yeast infections, and trichomoniasis. Accurate diagnosis using the Aptima vaginal panel supports better guided drug treatment, fewer recurrent doctor’s office visits, and reduced suffering in affected women. The Aptima® BV and Aptima® CV/TV assays usher in a new era of molecular testing, offering a comprehensive and accurate diagnosis of vaginosis and vaginitis.
Establishing a new standard for diagnosing vaginitis.
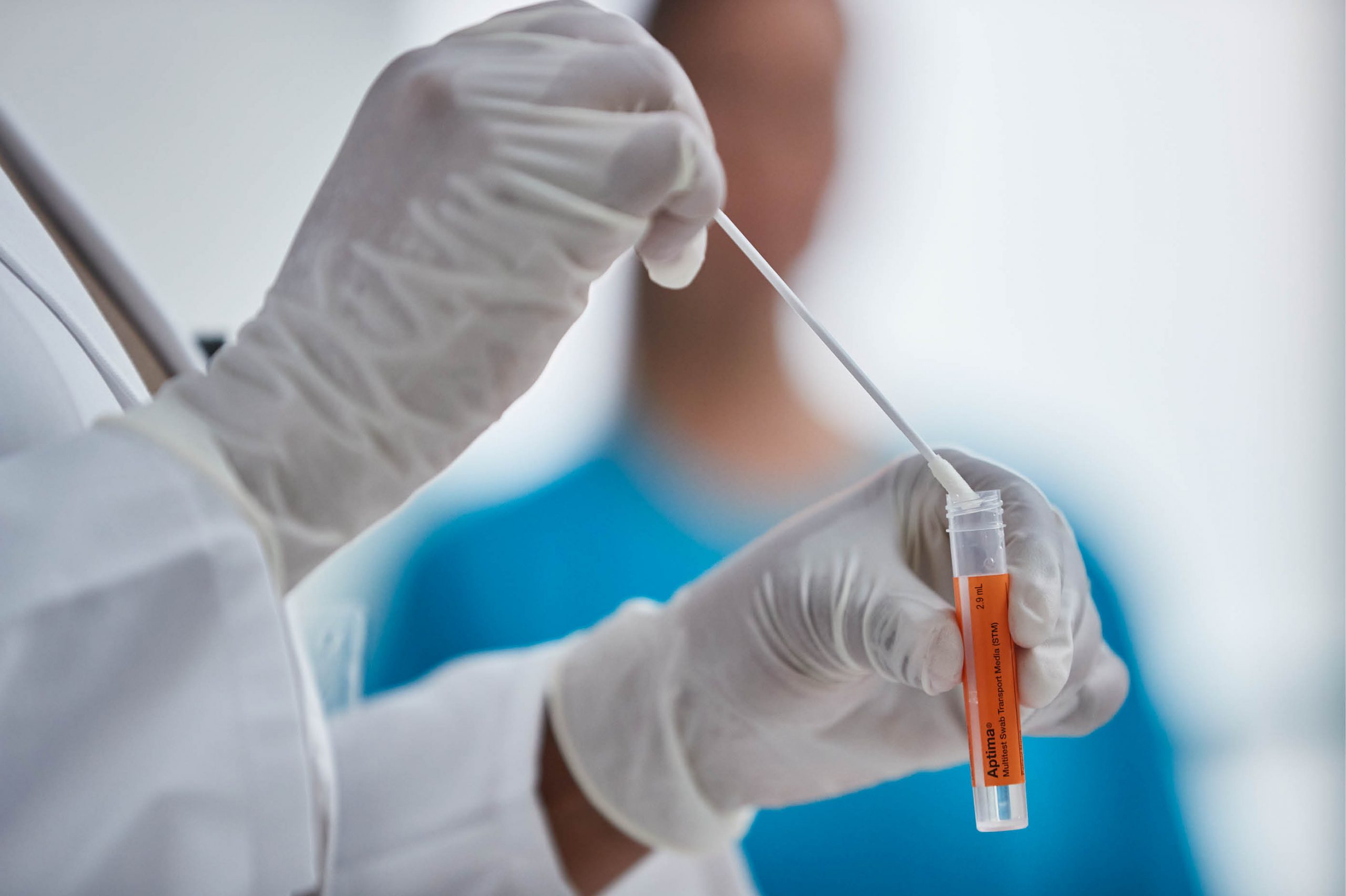
The Aptima BV and Aptima CV/TV assays are indicated for use in symptomatic women to aid in the detection of BV, Candida species, Candida glabrata, and Trichomonas vaginalis on the Panther® system. Specimens must be collected with the familiar orange Aptima Multitest Swab Specimen Collection Kit, via either clinician-collected or patient-collected vaginal swabs. As with our other Aptima diagnostic assays, one vaginal swab specimen can yield multiple test results on the automated Panther system.
Bacterial Vaginosis

The prevalence of bacterial vaginosis or “BV” in the United States is estimated at 21 million among women ages 14–49. Healthcare practitioners often overlook the association of untreated BV infections with serious consequences, including pelvic inflammatory disease, cervicitis, higher risk of acquiring STIs (chlamydia, gonorrhea, HSV, HIV), spontaneous abortion, and preterm birth. The Aptima BV assay demonstrates excellent sensitivity and specificity, due to its proprietary algorithm and assay design, targeting Gardnerella vaginalis, Atopobium vaginae, and Lactobacillus species. A simple qualitative BV positive or negative result is returned.
Candida Vaginitis and Trichomonas Vaginalis
Commonly known as a yeast infection, vulvovaginal candidiasis (candida vaginitis, “CV”) is a result of an overgrowth of fungal organisms, usually Candida albicans. Yeast infections can also be caused by the azole-resistant strain Candida glabrata, which is prevalent 8-16% of the time and requires a different treatment pathway than C. albicans. The Aptima CV/TV assay differentiates between Candida species and C. glabrata and can help healthcare providers determine the most appropriate antifungal therapy for their patients.
Sometimes referred to as “trich”, TV is the most common curable STI in the United States. Left untreated, TV infection is associated with an increased risk of HIV acquisition and transmission, prolonged HPV infection, higher risk of acquiring STIs such as chlamydia, gonorrhea and HPV, and can lead to premature labor and low birth weight babies. The CDC recommends testing for TV in all women seeking treatment for vaginal discharge. The Aptima CV/TV assay meets the recommendations by the CDC for a highly sensitive and specific test for detecting TV.
One orange tube, one solution, maximum efficiency.
The Aptima vaginal panel rounds out Hologic’s Aptima assay portfolio for sexual and vaginal health, which now includes vaginitis testing alongside comprehensive STI and viral load testing. Labs and healthcare providers now have the flexibility to detect up to seven infections and disease states with just one vaginal swab, including BV, Candida species, C. glabrata, Trichomonas vaginalis, chlamydia, gonorrhea, and Mycoplasma genitalium.