Blood Group Genotyping
Blood group genotyping systems help determine blood groups in individuals who may not be able to be typed with traditional blood grouping methods due to recent transfusions, autoantibodies, or other limitations.
Blood group genotyping for human erythrocyte antigens overcomes blood grouping limitations by looking directly into the DNA sequence and thereby avoiding any donor cell or patient antibody interference. Additional benefits of blood group genotyping include improved transfusion compatibility and reduced alloimmunization.
TYPING
BLOODchip ID
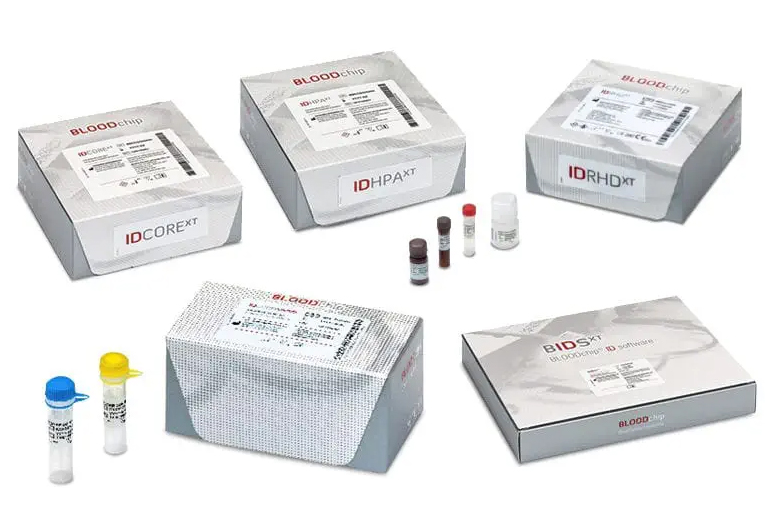
A multiplex blood group genotyping family of products based on Luminex technology; including kits for the main allelic variants of red blood cells (ID CORE XT, ID RHD XT), human platelet antigens (ID HPA XT), a positive control for ID CORE XT (ID CORE CONTROL), and an accompanying software (BIDS XT).

Efficient
Quickly progress from DNA to results with a fast and easy operating procedure.
- Only 4 tubes to pipette
- No washing or filtration steps
- Ready-to-use reagents
- Approximately 4 hours overall time from DNA to results
- Minimal hands-on time (approx. 30 minutes)
- Connect with Luminex to simplify the procedure
Flexible
Accommodate both donor and patient typing with BLOODchip ID’s throughput, flexibility, and antigen panels.
- From 1 to 96 samples per run
- Multiple product batches – ID CORE XT and ID HPA XT can be performed in the same run
- Open technology – standard Luminex equipment can be used for other products
- Multiple search functions – BIDS XT’s phenotype and genotype searching tools help find the appropriate units or patients
- Results by sample or batch of samples with reporting in multiple formats (.xls, .pdf)
Reliable
Support safer transfusion therapy for patients with a fast and effective solution.
- 100% accuracy1-3
- Accurate r’s testing in one single test
- High precision in reproducibility and repeatability2
- Management of positive and negative controls
Traceability
Keep track of activity with BIDS XT’s comprehensive database for samples, clinical information, and test results.
- Register all actions performed by users
- Plate configuration, kit and enzyme lot, and registration functions help users trace sample, reagents, and results
- Worksheet printouts with calculated volumes support users with the procedure
- Friendly and detailed reports and raw data graphs provide a detailed comprehension of results

